Page 51 - Heavenly Signs III by Mel Gable
P. 51
49
This would cause the ripping of hydrogen and oxygen atoms apart into elementary particles. This would begin a
thermonuclear fusion reaction. The reaction would produce intense light during the process and would generate
many different atomic nuclei. Remember during World War II, Germany was planning on using heavy water to
produce nuclear weapons. Nevertheless, it was a series of actions undertaken by Norwegian saboteurs during
World War II to prevent Germany from acquiring heavy water (deuterium oxide), which could have been used to
produce nuclear weapons. What does the Bible have to tell us about this transformation of water? This would
mean that at the instant of creation the earth was a small region of water at the center of a larger ball of water
which the Bible calls the “deep.” Dr. Russell Humphreys believes it to be a light-year in radius.
Nucleosynthesis
What does science have to say about this transformation of pure water? The modern word is nucleosynthesis to
describe this transformation of water into other elements. This would be heavy water normally called deuterium
2
oxide or H2O or D2O. Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons
(protons and neutrons). The first nuclei were formed about three minutes after what scientist call the Big Bang. It
was through the process called nucleosynthesis, which formed the hydrogen and helium content of the first
stars, and is responsible for the general hydrogen to helium ratio of the universe today. There are two important
characteristics of Big Bang nucleosynthesis (BBN). Please notice that for this nucleosynthesis event to occur it
74
took less than a day, of approximately 20 minutes.
It lasted for only about seventeen minutes (during the period from 3 to about 20 minutes from the beginning of space
expansion). After that, the temperature and density of the universe fell below that which is required for nuclear fusion. The
brevity of BBN is important because it prevented elements heavier than beryllium from forming while at the same time
allowing unburned light elements, such as deuterium, to exist.
It was widespread, encompassing the entire observable universe.
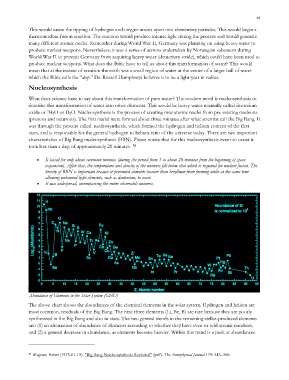
Abundance of Elements in the Solar System (GNU)
The above chart shows the abundances of the chemical elements in the solar system. Hydrogen and helium are
most common, residuals of the Big Bang. The next three elements (Li, Be, B) are rare because they are poorly
synthesized in the Big Bang and also in stars. The two general trends in the remaining stellar-produced elements
are: (1) an alternation of abundance of elements according to whether they have even or odd atomic numbers,
and (2) a general decrease in abundance, as elements become heavier. Within this trend is a peak at abundances
74 Wagoner, Robert (1973-01-15). "Big Bang Nucleosynthesis Revisited" (pdf). The Astrophysical Journal 179: 343–360.